

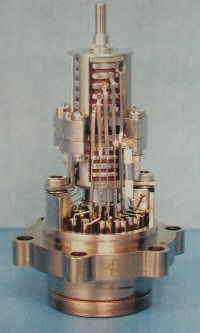
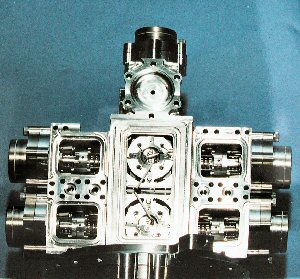
Once the gas sample is obtained, it is then converted into ions (charged particles) by bombarding the gas with an electron beam. Huygens' GCMS has two different energies that it can use for the electron beam. Using different energies allows the instrument to detect and analyze a larger variety of chemicals.
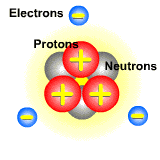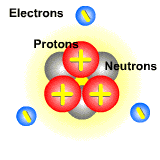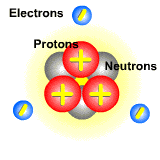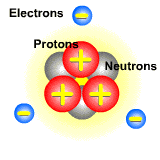
How does bombarding atmospheric gas with an electron beam change the gas into ions? The gas is comprised of molecules, and molecules are built from atoms. Atoms consist of electrons (negatively charged particles), neutrons (charge-less particles), and protons (positively charge particles). Atoms are electrically neutral; they have no charge because the number of electrons is the same as the number of protons.
Pictured below is an atom. The protons and neutrons are located in the center. They make up the nucleus of the atom. The electrons orbit around the nucleus. Since electrons are on the outside of the atom and are lighter than protons, they can easily be moved from atom to atom.
When the electron beam hits the atoms of a molecule, it dislodges an electron. The atom that lost the electron now has more protons than electrons, and this imbalance gives it a postive charge. These positively charged particles are ions called cations.
Once the electron has been dislodged, it may hit a different atom of a molecule. When the electron hits the atom, the atom captures it into its electron cloud, thus giving the atom more electrons than protons. The atom is the negatively charged, and these type of ions are called anions.